Dion Khodagholy, assistant professor of electrical engineering, is focused on developing bioelectronic devices that are not only fast, sensitive, biocompatible, soft, and flexible, but also have long-term stability in physiological environments such as the human body. Such devices would greatly improve human health, from monitoring in-home wellness to diagnosing and treating neuropsychiatric diseases, including epilepsy and Parkinson’s disease. The design of current devices has been severely constrained by the rigid, non-biocompatible electronic components needed for safe and effective use, and solving this challenge would open the door to a broad range of exciting new therapies.
In collaboration with Jennifer N. Gelinas, Department of Neurology, and the Institute for Genomic Medicine at Columbia University Iriving Medical Center, Khodagholy has recently published two papers, the first in Nature Materials (March 16) on ion-driven soft and organic transistors that he and Gelinas have designed to record individual neurons and perform real-time computation that could facilitate diagnosis and monitoring of neurological disease.
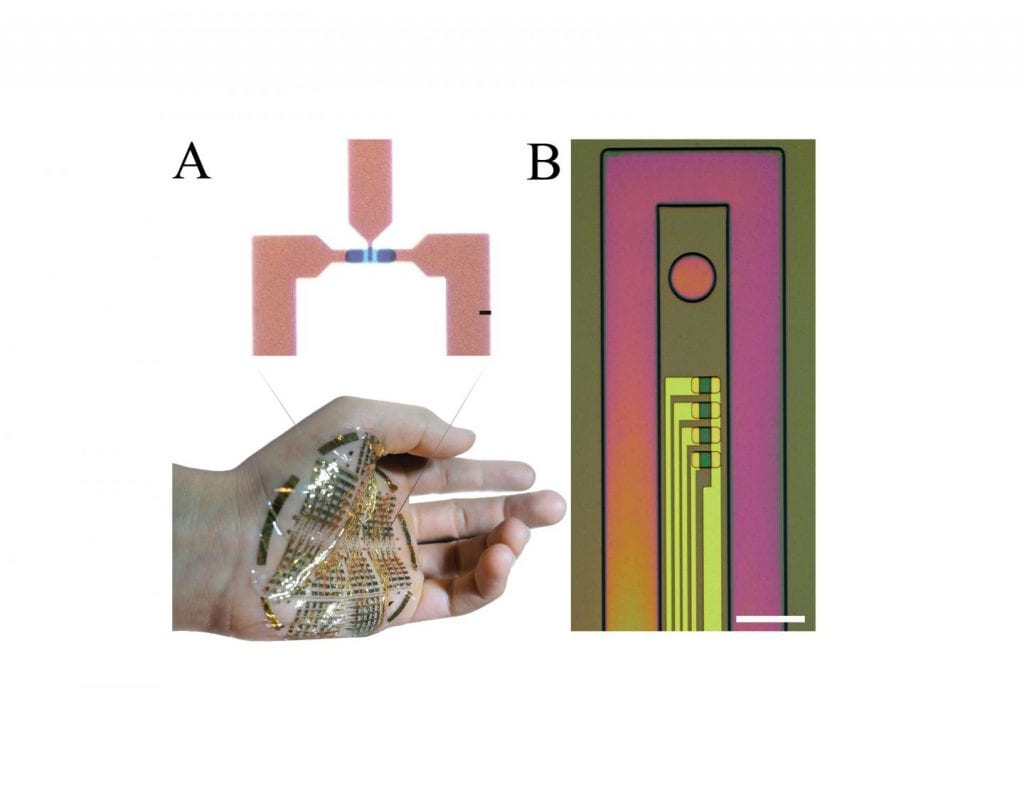
The second paper, published today in Science Advances, demonstrates a soft, biocompatible smart composite–an organic mixed-conducting particulate material (MCP)–that enables the creation of complex electronic components which traditionally require several layers and materials. It also enables easy and effective electronic bonding between soft materials, biological tissue, and rigid electronics. Because it is fully biocompatible and has controllable electronic properties, MCP can non-invasively record muscle action potentials from the surface of arm and, in collaboration with Sameer Sheth and Ashwin Viswanathan at Baylor College of Medicine’s department of neurosurgery, large-scale brain activity during neurosurgical procedures to implant deep brain stimulation electrodes.
“Instead of having large implants encapsulated in thick metal boxes to protect the body and electronics from each other, such as those used in pacemakers, and cochlear and brain implants, we could do so much more if our devices were smaller, flexible, and inherently compatible with our body environment,” says Khodagholy, who directs the Translational NeuroElectronics Lab at Columbia Engineering. “Over the past several years, my group has been working to use unique properties of materials to develop novel electronic devices that allow efficient interaction with biological substrates–specifically neural networks and the brain.”
Conventional transistors are made out of silicon, so they cannot function in the presence of ions and water, and in fact break down because of ion diffusion into the device. Therefore, the devices need to be fully encapsulated in the body, usually in metal or plastic. Moreover, although they work well with electrons, they are not very effective at interacting with ionic signals, which is how the body’s cells communicate. As a result, these properties restrict the abiotic/biotic coupling to capacitive interactions only on the surface of material, resulting in lower performance. Organic materials have been used to overcome these limitations as they are inherently flexible, but the electrical performance of these devices was not sufficient to perform real-time brain signal recording and processing.
Khodagholy’s team took advantage of both the electronic and the ionic conduction of organic materials to create ion driven transistors they call e-IGTs, or enhancement-mode, internal ion-gated organic electrochemical transistors, that have embedded mobile ions inside their channels. Because the ions do not need to travel long distances to participate in the channel switching process, they can be switched on and off quickly and efficiently. The transient responses depend on electron hole rather than ion mobility, and combine with high transconductance to result in a gain-bandwidth that is several orders of magnitude above that of other ion-based transistors.
The researchers used their e-IGTs to acquire a wide range of electrophysiological signals, such as in vivo recording of neural action impulses, and to create soft, biocompatible, long-term implantable neural processing units for the real-time detection of epileptic discharges.
“We’re excited about these findings,” says Gelinas. “We’ve shown that E-IGTs offer a safe, reliable, and high-performance building block for chronically implanted bioelectronics, and I am optimistic that these devices will enable us to safely expand how we use bioelectronic devices to address neurologic disease.”
Another major advance is demonstrated by the researchers in their Science Advances paper: enabling bioelectronic devices, specifically those implanted in the body for diagnostics or therapy, to interface effectively and safely with human tissue, while also making them capable of performing complex processing. Inspired by electrically active cells, similar to those in the brain that communicate with electrical pulses, the team created a single material capable of performing multiple, non-linear, dynamic electronic functions just by varying the size and density of its composite mixed-conducting particles.
“This innovation opens the door to a fundamentally different approach to electronic device design, mimicking biological networks and creating multifunctional circuits from purely biodegradable and biocompatible components,” says Khodagholy.
The researchers design and created mixed conducting particulate (MCP)-based high performance anisotropic films, independently addressable transistors, resistors, and diodes that are pattern-free, scalable, and biocompatible. These devices carried out a variety of functions, including recording neurophysiologic activity from individual neurons, performing circuit operations, and bonding high-resolution soft and rigid electronics.
“MCP substantially reduces the footprint of neural interface devices, permitting recording of high-quality neurophysiological data even when the amount of tissue exposed is very small, and thus decreases the risk of surgical complications,” says Gelinas. “And because MCP is composed of only biocompatible and commercially available materials, it will be much easier to translate into biomedical devices and medicine.”
Both the E-IGTs and MCP hold great promise as critical components of bioelectronics, from wearable miniaturized sensors to responsive neurostimulators. The E-IGTs can be manufactured in large quantities and are accessible to a broad range of fabrication processes. Similarly, MCP components are inexpensive and easily accessible to materils scientist and engineers. In combination, they form the foundation for fully implantable biocompatible devices that can be harnessed both to benefit health and to treat disease.
Khodagholy and Gelinas are now working on translating these components into functional long-term implantable devices that can record and modulate brain activity to help patients with neurological diseases such as epilepsy.
“Our ultimate goal is to create accessible bioelectronic devices that can improve peoples’ quality of life,” says Khodagholy, “and with these new materials and components, it feels like we have stepped closer to that.”