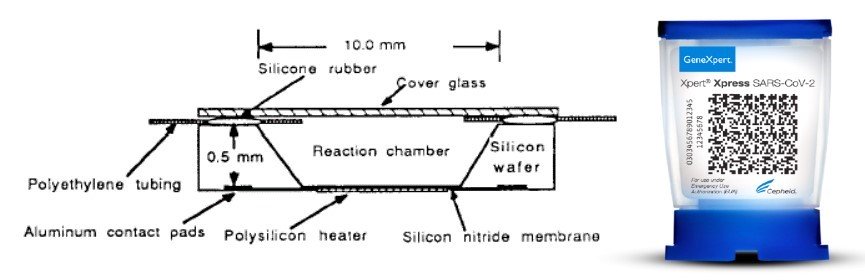
On Saturday, March, 21, 2020 the U.S. Food and Drug Administration (FDA) gave emergency authorization to Cepheid, a California company, to sell a new test for rapid detection of the pandemic coronavirus SARS-CoV-2, which causes COVID-19. Cepheid’s Xpert® Xpress SARS-CoV-2 test gives healthcare workers results in just 45 minutes, with less than a minute of hands-on time for sample preparation.
Cepheid, founded by Kurt Petersen, M. Allen Northrup and five others in 1996, is well known in the MEMS community for commercializing microfluidic chip-based polymerase chain reaction (PCR) analysis machines. This is not the first time Cepheid has responded quickly to a biological threat; after the 2001 terrorist attacks in the USA, Cepheid was the first to provide rapid anthrax detection capabilities to the U.S. Postal Service, and it still does today.
At the heart of all COVID-19 test protocols (see the WHO protocol and U.S. CDC protocol) is the real-time reverse transcription polymerase chain reaction (RT-PCR) analysis technique. In a very simplified description, PCR uses thermal cycling to amplify the DNA present in a patient’s swab sample, and then using fluorescence optical detection, searches for the virus’s specific DNA. The test requires knowing the virus’s genome in the first place; the crucial work to sequence the full genome of SARS-CoV-2 was first published by Chinese scientists for public use on January 10, 2020.
While traditional PCR machines take many hours to thermal cycle and reach a result, MEMS-based PCR systems can work much faster. Featuring scale heaters and reaction chambers that have a tiny thermal mass, they create a significantly faster heat-cool cycle, enabling a rapid result in minutes.
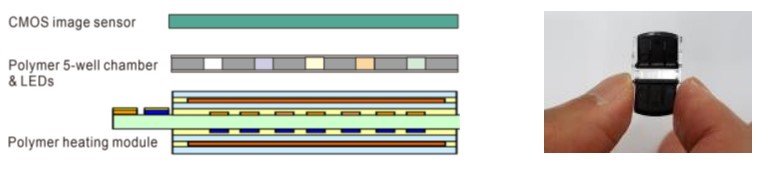
Research on MEMS-based PCR systems has continued steadily since the early 1990s. Today, researchers have been focusing on developing highly integrated, low-cost systems specifically for point-of-care use. One example of recent research: a team at Korea’s ETRI and Genesystem have developed a prototype low-cost, handheld PCR system having a polyimide chamber and microheater and an integrated CMOS detector for optical readout of results (figure below).
Korea’s quick recruitment of its biotech companies and creation of novel drive-through testing sites helped it to successfully pinpoint its COVID-19 outbreak and to implement control measures. Let’s hope the Cepheid test can be similarly effective.
Based on successive epidemics of SARS, MERS and now COVID-19, rapid PCR test machines, enabled by MEMS technology, are becoming essential medical tools in the fight against viral outbreaks. As continued development lowers the cost of such critical equipment, let’s hope we may soon have a PCR machine in every doctor’s office.