Semiconducting polymers, very large chain-like molecules made from repeating sub-units, are increasingly drawing the attention of researchers because of their potential applications in organic electronic devices. Like most semiconducting materials, semiconducting polymers can be classified as p-type or n-type according to their conducting properties. Although p-type semiconducting polymers have seen dramatic improvements thanks to recent advances, the same cannot be said about their n-type counterparts, whose electron-conducting characteristics (or ‘electron mobility’) are still poor.
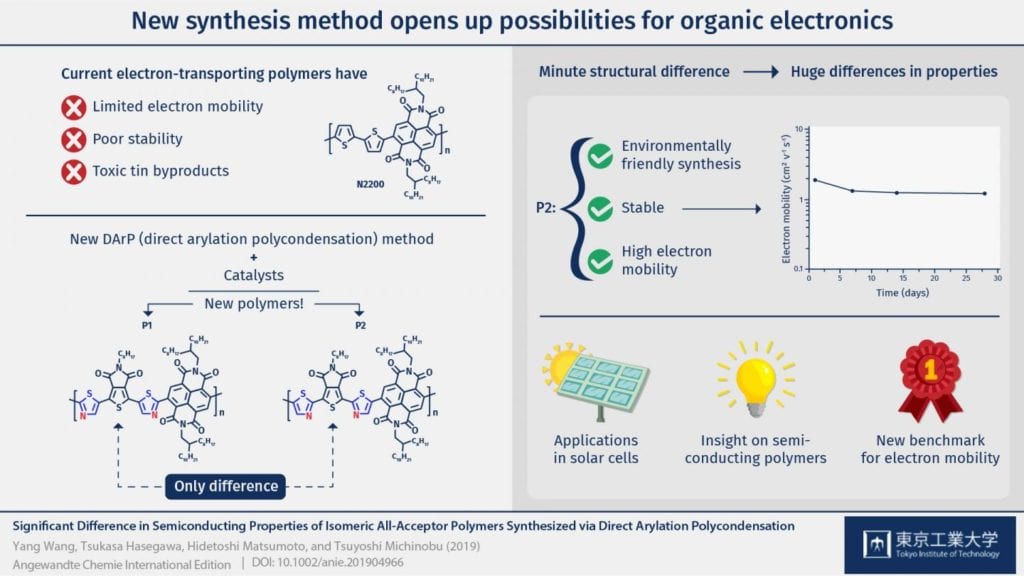
Unfortunately, high-performance n-type semiconducting polymers are necessary for many green applications, such as various types of solar cells. The main challenges holding back the development of n-type semiconducting polymers are the limited molecular design strategies and synthesis procedures available. Among the existing synthesis methods, DArP (which stands for ‘direct arylation polycondensation’) has shown promising results for producing n-type semiconducting polymers in an environmentally friendly and efficient way. However, until now, the building blocks (monomers) used in the DArP method were required to have an orienting group in order to produce polymers reliably, and this severely limited the applicability of DArP to make high-performance semiconducting polymers.
Luckily, a research team from Tokyo Institute of Technology led by Prof. Tsuyoshi Michinobu found a way around this. They managed to reliably produce two long n-type semiconducting polymers (referred to as P1 and P2) through the DArP method by using palladium and copper as catalysts, which are materials or substances that can be used promote or inhibit specific reactions.
The two polymers were almost identical and contained two thiazole rings-pentagonal organic molecules that contain a nitrogen atom and a sulfur atom. However, the position of the nitrogen atom of the thiazole rings was slightly different between P1 and P2 and, as the researchers found out, this led to significant and unexpected changes in their semiconducting properties and structure. Even though P1 had a more planar structure and was expected to have a higher electron mobility, it was P2 who stole the show. The backbone of this polymer is twisted and looks similar to alternating chain links. More importantly, the researchers were surprised to find that the electron mobility of P2 was forty times higher than that of P1 and even higher than that of the current benchmark n-type semiconducting polymer. “Our results suggest the possibility of P2 being the new benchmark among n-type semiconducting materials for organic electronics,” remarks Prof. Michinobu.
In addition, semiconducting devices made using P2 were also remarkably stable, even when stored in air for a long time, which is known to be a weakness of n-type semiconducting polymers. The researchers believe that the promising properties of P2 are because of its more crystalline (ordered) structure compared with P1, which changes the previous notion that semiconducting polymers should have a very planar structure to have better semiconducting properties. “Our new DArP method opens a door for synthesizing various promising n-type semiconducting polymers which cannot be obtained via traditional methods,” concludes Prof. Michinobu. This work is another step in the direction towards a greener future with sustainable organic electronics.